INTRODUCTION
The African Medicines Regulatory Harmonization (AMRH) Joint Secretariat has been working with the African Medical Devices Forum (AMDF) Technical Committee to update the list of COVID-19 diagnostic tests and medical devices including personal protective equipment (PPEs). This is a list of products which have been authorized/listed by WHO EUL and other institutions to inform National Regulatory Authorities (NRA) with an objective of facilitating in country authorization/registration of the listed products for clinical diagnosis of COVID 19 or research and epidemiology purposes. The list is submitted to the AMRH Steering Committee for endorsement and subsequently to be shared with all NRA in the African countries.
LIST OF COVID-19 IN VITRO DIAGNOSTIC TESTS
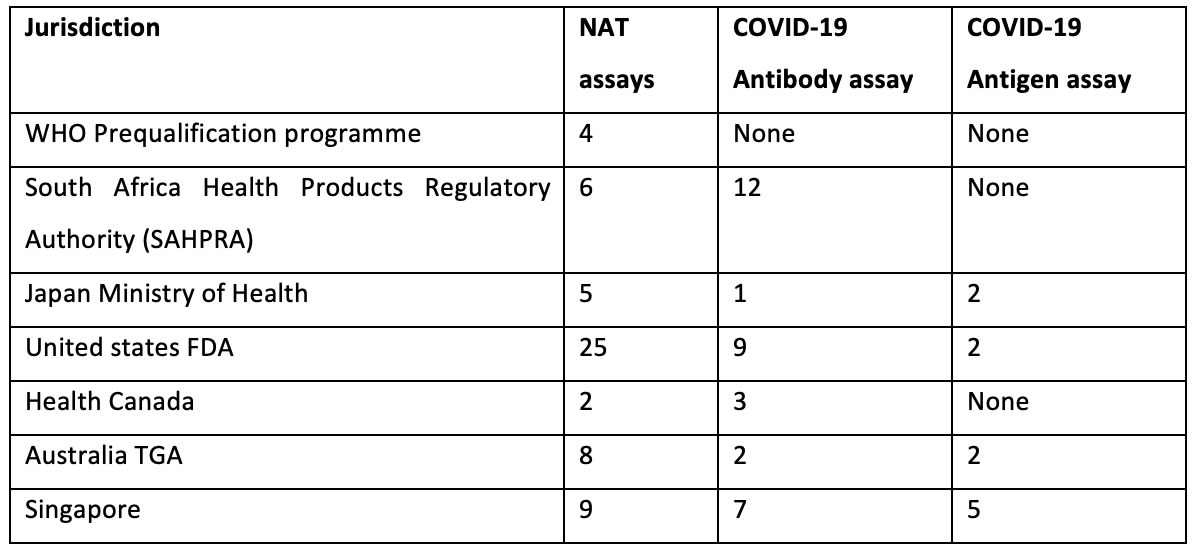
The number of COVID-19 in vitro diagnostics which have been authorized during the month of August and September 2020 by various jurisdiction for Emergency use during the current epidemic is summarized in the Table below.
MEDICAL DEVICES AND MANUFACTURERS
Lists of registered medical devices in African countries, IMDRF countries and African (local) COVID 19 manufacturers of COVID-19 medical devices have been updated to include the information as shown below:
- COVID19 related medical devices authorized by NRAs of Zimbabwe and South Africa.
- List of domestic manufacturers of COVID-19: Ethiopia, Botswana and Kenya and South Africa.
- List of COVID19 medical devices approved by IMDRF member states and links to N95 masks, Respirators, and surgical masks authorized by the US FDA.
The documents are presented as annex 1 and 2 and attached to this summary report.




